Innovative learning and performance solutions.
GMP training and workshops



LearningPlus workshops provide current expectations on GMP topics along with unique case studies and activities where participants can work and learn together. Each onsite/inhouse workshop is adapted to meet the specific needs of the organization. Our most popular, current workshops are below. Download the pdf for more details. Public workshops are presented in conjunction with organizations like Key2Compliance (Northern Europe), FDANews (U.S.), PDA (U.S.), and PSG (Canada).
Quality Risk Management (QRM) Fundamentals and Tools
Regulatory agencies world-wide are encouraging our industry to identify, assess, and manage the potential quality risks associated with pharmaceutical and biotech products. Pharmaceutical firms are also realizing that risk management is an important way to rationally set priorities for quality and compliance activities. Download PDF: QRM Two-day Course description May 2015
Procedures for Performance
Procedures are an important way of describing how a task is to be performed so personnel can perform the task correctly, consistently, safely, and efficiently. Despite their critical role in making pharma and biopharma products as well as medical devices, writing a clear, easy to follow procedure – and then training people on it – isn’t as simple as it sounds. This two-day workshop looks at procedures as one of several methods for managing, capturing, and transferring knowledge. Various formats of procedures will be examined and critiqued along with an examination of current regulatory requirements. Using a unique data collection form, participants will collect information that would be included in a procedure and then write (or re-write) a procedure. Participants will have an opportunity of writing (or re-writing) a procedure based on an actual need they have. Download PDF: Procedures for Performance Two-day Course description
Better Investigations, Corrections, and Corrective Actions
Problems like deviations and failures are a fact of life but individuals and organizations that understand what happened and why are in a much stronger position to establish corrective actions to reduce the likelihood of the event happening again. This two-day workshop examines a defined, logical process that can be applied to investigations that are performed in the drug, biotech, and medical device industries. Tools like data collection sheets, checklists, and interview worksheets, based on some of the best practices in a variety of industries are provided and used. Various problem solving, interviewing, and data collection techniques are also presented. Download PDF: Better Investigations, Better Corrective Actions
Leading the Climb: Seven essentials of GMP
Leading the Climb is an advanced GMP training course targeted at those who have supervisory/management responsibilities, those working in quality, compliance, training, and other staff/technical positions. The course covers GMP expectations of the US, Canada, Europe, and the World Health Organization (WHO). The course uses a unique video produced by LearningPlus that builds an analogy of mountain climbing and being a guide. Role modeling, systems thinking, coaching and feedback, and satisfaction in completing a challenging task are discussed. Activities that involve collaboration and teamwork allow participants to apply their experience and newly-gained knowledge to challenging situations as they develop answers using “GMP thinking.” Participants use (and keep) a resource book containing a variety of regulatory reference documents, warning letters, and articles. The course is annually updated to reflect revised FDA enforcement data, recent warning letters, and current industry/agency issues. At the end of the course, a case study is used to illustrate the business, personal, and financial impact of not meeting GMPs. Leading the Climb has been used in more than three dozen pharmaceutical companies. It has been adopted by a multinational pharmaceutical company as one of several required courses in its management curriculum; LearningPlus has trained over 5000 of its personnel since 1998. The course has been used and well received in the US, Canada, Singapore, Puerto Rico, Italy, and Ireland. Download PDF: Leading the Climb description and objectives 2014
GMP Update
Each year, LearningPlus revises its popular GMP Update course with information about new and revised regulations, guidances, and expectations from the FDA and regulatory agencies in Canada and Europe. Designed for quality staff, support professionals, and management at all levels, this 3-hour workshop also includes compliance and inspection trends, cutting-edge topics, and a case study where participants can apply their knowledge and critical “GMP thinking” skills. Download PDF: GMP Update
Learning, Knowledge Management, and Impact: Moving from Theory to Practice
Organizations in the pharmaceutical and other industries are changing from a simple “training” model to one that emphasizes learning. This fundamental shift affects everyone in the organization, providing many more formal and informal ways to acquire and distribute knowledge and develop skills. This course examines how a learning-focus can help in the acquisition, distribution, and use of knowledge – something viewed by regulatory agencies as a fundamental enabler of a modern quality system. Practical examples are shared and solutions developed. Download PDF: Learning, Knowledge Management, and Impact
GxP Basics
GxP Basics presents a broad overview to regulations (GLPs, GCPs, GMPs, GDPs, and QSR) that apply to non-clinical testing, clinical testing, manufacturing, distribution, and medical devices. This three-hour course provides a history and essentials of each regulation. This course can include information on the life cycle of a drug product and key activities that both regulatory agencies and the drug firm/sponsor perform. The course has been presented to all levels of personnel within a firm and also in an academic/university medical center setting. Download PDF: GXP Basics
Management Responsibilities in GMP Environment
Targeted for senior management/leadership and also used with boards-of-directors, the Management Responsibilities course presents an overview of the drug laws and regulations that apply to drug manufacturing organizations. Examples of non-compliance (e.g., Warning Letters and Consent Decrees) are discussed with the intent of showing what can happen if effective quality systems and management oversight are not present. The course is typically adapted to the specific needs/interests of the learners. Download PDF: Management Responsibilities in a GMP Environment
Consulting and customized solutions



Much of LearningPlus’ work applies our knowledge, skills, and experience to an organization’s specific problem or project. As needed, LearningPlus expands the team with other experts in a field such as microbiology, assessment and evaluation, instructional design, and statistics.
Baseline GMP/QSR training
To forestall a significant regulatory action, LearningPlus was asked to create a 3-4 hour “baseline” course covering drug GMPs and the medical device QSRs for a multi-national organization. The content is being presented using the firm’s instructors. Capability for local adaptation (for products and specific regulatory concerns) was a key feature of the design. The course will be seen by over 35,000 personnel world-wide.
Contamination Control and Vaccine Technology course instruction/facilitation
To support a vaccine manufacturer’s internal training efforts, Dr. Vesper was asked to join the firm’s cadre of instructors to co-teach a number of sessions and provide a broader industry perspective on current practices. Courses have been conducted at several US sites and in Ireland, Belgium, France, Mexico, and Japan.
Needs analysis and program design
A manufacturer of sterile injectable products asked LearningPlus to conduct a detailed needs analysis and high-level design document for a knowledge course on contamination control and a follow-up “hands-on” practical course for those working in aseptic areas. This project was part of a US FDA Warning Letter remediation commitment.
Global benchmarking of learning programs
A large manufacturing organization that was rapidly expanding wanted information on the current training/learning practices at its sites. LearningPlus developed and implemented a comprehensive survey and set of phone interviews with operations personnel, training staff, and management. A detailed report and presentation was produced that gave the firm insights into the strengths and weaknesses of its compliance and operations training efforts.
Serious games and customized e-learning


E-learning is being used by more and more organizations in providing information and training. Unfortunately, most e-learning is not much more than power-point slides with sound and colorful graphics. LearningPlus has developed a variety of e-learning courses that are use the web and personal technology in unique ways.
GMP e-Update
Several firms have come to LearningPlus and asked it to create an on-line version of its popular workshop. Topics important to the firm were included as well as information from regulatory agencies like U.S. FDA.


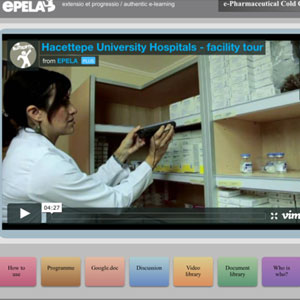
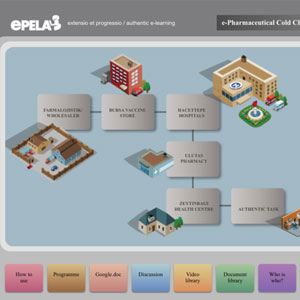
WHO e-Pharma Cold Chain Management
LearningPlus helped design this award-winning e-learning course that extends over 12 weeks. The course is modeled after an actual physical course conducted every year in Turkey. Several technical papers about this unique course have appeared in leading pharmaceutical and learning publications.

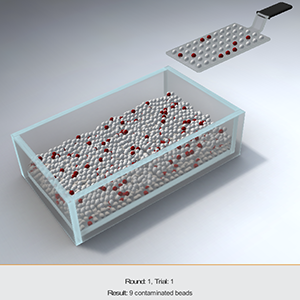
GAMES!
The newest area for LearningPlus is an active collaboration with game developer, BrokenMyth Studios. Serious games are a way to involve the learner in a challenging but fun simulation or activity.
-
Sterility Sampling Statistical Simulator (S4)
The Sterility Sampling Statistical Simulator (S4) is a tool that can be used in training people in statistically-based sampling and specifically in the limitations of the sterility test used by the pharmaceutical and medical device industries.S4 can be used by individuals, small groups, and in larger instructor-led training events. Users can select from total population options, defect/contamination rates, and sampling sizes, including the sampling size of 20 units as required by many regulatory agencies. Coming soon to the Apple App Store and Android Play.

Examples of work



Since 1992, LearningPlus worked on a large number of fascinating and challenging projects around the world contributing to drug products that are safe, effective, and available. Here are some examples of what we’ve done.
Training for Chinese SFDA
LearningPlus was invited to be part of a capacity-building project to support the Chinese SFDA develop its capabilities in performing Good Clinical Practice (GCP) inspections. English-speaking inspectors participated in a special facilitation skills course that helped them effectively adapt and then teach a GCP learning course for their Chinese-speaking colleagues.

Establishing learning systems and quality/GMP courses for a new site
As part of a long-term plan to have its products approved in the U.S. and Europe, biologics firm in Asia requested that LearningPlus create an integrated curriculum for all its personnel and management team members. The learning events that LearningPlus will present start with basic GMP and then progresses to topics like procedures, recordkeeping, risk management, and investigations. Most courses are in the form of workshops and include authentic activities, such as doing actual investigations and report writing, and conducting risk assessments on operations and activities.

Workshop on management responsibilities
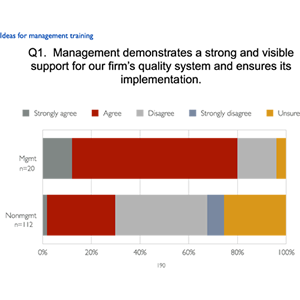
For a pharma firm’s annual GMP program for management, LearningPlus developed a survey to better understand how management and operations personnel each viewed management’s commitment to the ICH Q10 view of quality. The questions were based on specific Q10 statements. The results, along with possible reasons and ways to better communicate management’s quality vision were discussed by the participants. Other aspects of the workshop looked at current cases of noncompliance, highlighting the impacts on the finances and reputation of the organization.
Training System Assessment and Recommendations
A biotechnology firm wanted an outside expert evaluation of its training system in light of its rapid expansion and quality goals. LearningPlus conducted interviews with management and other stakeholders, developed questionnaires, and used site visits and observations to provide an assessment of the current program’s strengths and weaknesses. The solution included a high-level curriculum design on quality/compliance topics as well as recommendations to help build formal and informal learning practices into the fabric of the organization.
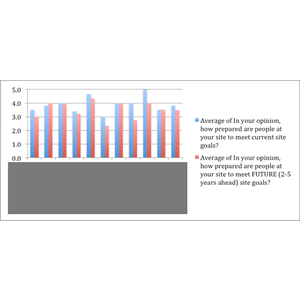
For more examples of projects that involved LearningPlus download these PDFs:



Often, at the end of a workshop, participants (anonymously) complete course evaluations. Here are some that we’ve received.
What can we do for you?


LearningPlus has worked with a number of small and large pharma and biopharma firms world-wide. We have a specific focus on projects related to GMP performance and topics like risk management, investigations/corrective actions, course design, and learning systems. Each project is unique in that the solution must meet organization-specific goals and also integrate with the organization’s culture and work practices. We would welcome the opportunity of talking with your organization and learning ways that we can use our experience and skills to assist with your training and performance needs.
Comments from clients and participants